Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

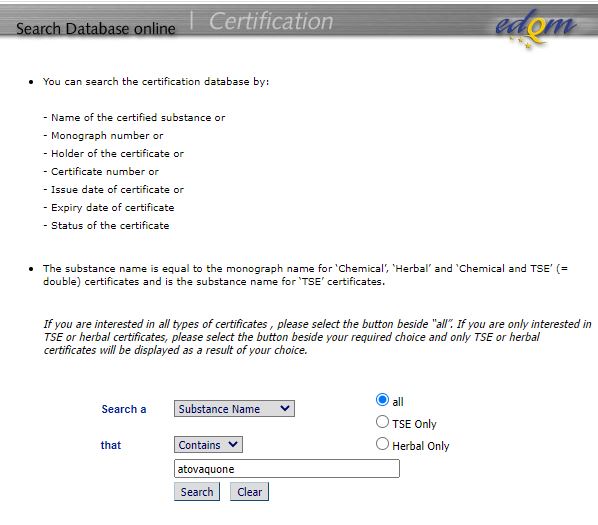
An Overview by Saravanaraja Subramanian 1. Risk & Regulations of TSE/BSE in pharmaceuticals are of great importance because of its irreversible fatal. - ppt download
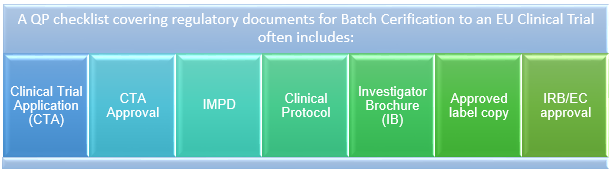
Managing Clinical Trial Application (CTA) Acceptability to Support Phase I Clinical Studies in the United Kingdom
Clinical trial registration, reporting, publication and FDAAA compliance: a cross-sectional analysis and ranking of new drugs ap